IceCure uses probes to freeze tumors, killing the tissue. The tumor then disperses naturally.
ILANIT CHERNICK
IceCure, a Tel Aviv-based medical device company has received additional US Food and Drug Administration approval for its tumor-treating cryoablation technology.
In an interview with The Jerusalem Post on Thursday, Tlalit Bussi Tel Tzure, IceCure’s vice president of business development and global marketing, said the company received its first approval by the FDA in 2010 following clinical trials and evaluations. The company was founded in 2006.
“The cryoablation system only became commercial in 2014,” she explained. “We had already general FDA approval to treat benign and cancerous tissues, but we didn’t have a specific application, meaning which organ we are treating with the cryoablation system.”
Tel Tzure explained the new FDA approval “allows us to expand our capabilities in the US, meaning we can now treat liver and kidney tumors that are cancerous with our system.”
IceCure also received approval for ear, nose and throat application, “but we are not active in that application yet.”
Asked how the system works, Tel Tzure explained that cryoablation is the destruction of the cancerous or benign tumor tissue by applying to it a very cold temperature.
“We use liquid nitrogen in order to decrease the temperature at the tip of a probe, and we insert the probe into the body – into the tumor – under imaging,” she explained. “For example if done for a breast tumor, it will be done with an ultrasound, so that the doctor will be able to see the tumor and know where to insert the probe.”
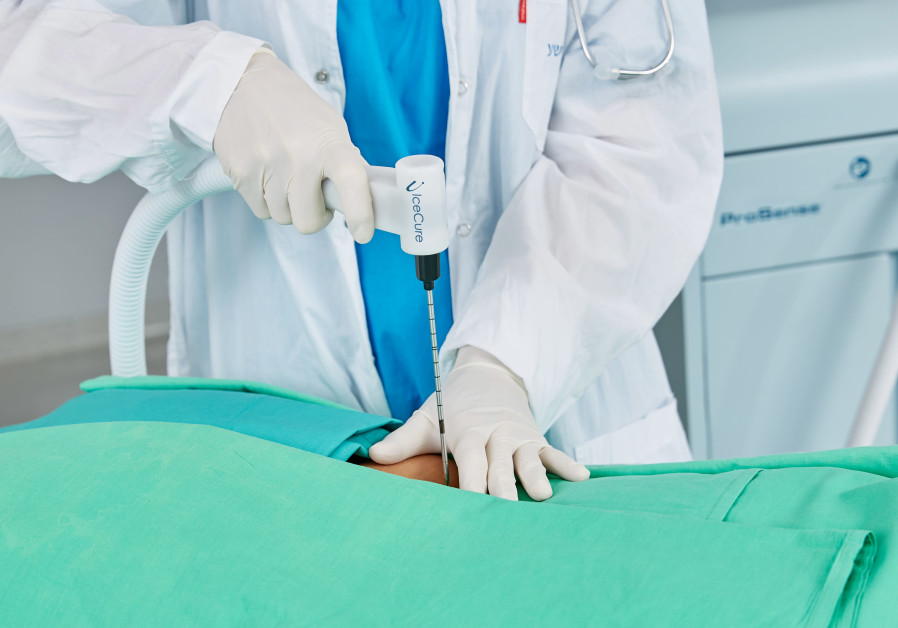
Once the probe is inside the tumor, she added, “then we use our system to decrease the temperature up to minus 170 degrees and it’s such a low temperature that the tissue is destroyed.
“When the tissue is destroyed, it dissolves out of the body naturally in a couple of weeks,” Tel Tzure continued.
The treatment time is short with procedures generally taking less than an hour.
“We can kill a tissue, whether it’s benign or cancerous, and we are avoiding surgery, so we are offering a minimally invasive treatment to kill a tumor by freezing,” Tel Tzure said. “It’s done with local anesthesia, there is no need to put the patient to sleep, it doesn’t cause any pain, there is no scarring, and also the patient doesn’t have to stay in the hospital to recover.”
Following the procedure, patients can get back to their daily activities on the same day.
She pointed out that for breast tumors in the US, IceCure has FDA clearance to treat benign tumors only, “which are called fibroadenomas and this is an office-based procedure that we are already doing.”
A fibroadenoma, or benign breast tumor, is something that usually happens to younger patients, causing pain and also changing the shape of the breast.
Tel Tzure added that the procedure for these usually takes less than half an hour.
However, cryoablation treatment for kidney and liver needs to be done in a CT room because they are inner organs and the doctors need to use CT imaging to see the tumor and to know where to insert the probe. “But again, there is no need for general anesthesia, no long hospital stay and here we are treating cancer patients, whereas for the breast it’s benign tumors.”
Asked about the size of tumors that can currently be treated with this technology, she said that breast tumors up to the size of 1.5 cm. can be treated, while a tumor in the kidney and liver can be treated up until 3 cm.
Asked about targeting larger tumors, Tel Tzure said that with the FDA clearance they received, IceCure got approval for their multi-sense system, “which uses three probes at the same time.”
She estimated that it would be available commercially by the end of 2021.
“This new system will allow us to treat much larger tumors and use multiple probes in order to make the treatment for larger tumors quick,” Tel Tzure added. During 2020, IceCure hopes to extend its activity in the US now that the company has this new FDA clearance and can offer its treatment to a larger population, especially as the number of cancer cases continues to rise.
“We can expand our activity by offering better care for more patients around the globe as well.”
IceCure, Tel Tzure said, is also being used in Europe and Asia.
“In Europe we have the CE mark, which means we can treat breast cancer patients and treat cancerous breast tumors, while in the US we still don’t have FDA approval for this,” she added. “In Japan, we are treating lung cancer and bone cancer patients as well.”
In Israel, the use of cryoablation technology is just beginning.
“We have a system available in two hospitals in Haifa,” Tel Tzure highlighted. “At Bnai Zion Medical Center, we are doing a clinical trial on kidney cancer patients, we have treated more than 100 with very good results.”
The system is also available in Elisha Hospital in Haifa, as well as in Hillel Yaffe Medical Center in Hadera and Ziv Medical Center in the North.
“We are looking to extend our activity in Israel as well,” she added.
Content retrieved from: https://www.jpost.com/HEALTH-SCIENCE/Israeli-tech-firm-specializing-in-freezing-tumors-gains-FDA-approval-in-US-613687?fbclid=IwAR3xmroW9_wiFAtOuMhHdx-7ATMFDhtSvM3SAR1aTNPbpggc1z_Ro__gpKU.